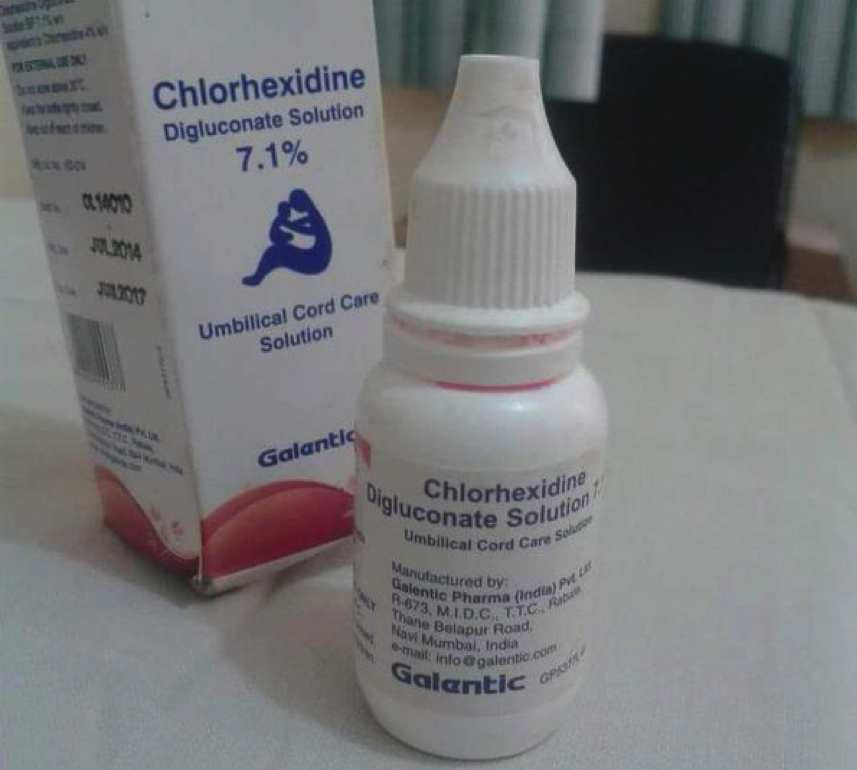
What are the consequences so far? There are five known cases of irreversible blindness associated with the use of these drops already, three reported in Yobe State and two in Adamawa State. The three children in Yobe are 3 weeks, 4 weeks and two years. Old. All their parents were uneducated, their fathers, farmers. This will represent the socio economic circumstances of many people in the area. An unknown number of these containers were distributed to patients in Yobe, Adamawa through government primary healthcare centres. The source of these bottles is not yet clear. They do not have a NAFDAC number inscribed on them. It is not clear if they were distributed in other states in Nigeria. We have some very graphic pictures of the consequences of these drops on children, which we have decided not to show here.
What has been the response? Many astute and hardworking physicians and other health care workers have personally led advocacy campaigns and search efforts to retrieve as many of these bottles still in circulation. Our understanding is that the Federal Ministry of Health and its agencies; National Agency for Food and Drug Administration (NAFDAC), whose vision is to “safeguard the health of the nation” and the National Primary Health Care Development Agency (NPHCDA) have responded to this, as well as the Yobe State Government, trying to find and retrieve as many of the remaining bottles in circulation. The Yobe State Ministry of Health has reached out to all the Primary Health Care and Maternal and Child Health coordinators in the Local Government Areas with relevant information and apparently all the remaining stock of the drugs have been collected and as much as possible, those that were already dispensed traced and retrieved.
It is clear that health workers did not actually prescribe the drugs for use in eyes but the packaging was misleading and parents mistakenly used these as eye drops. Local awareness campaigns have been carried out and the three children affected in Yobe are getting care and support. At the time of publishing this piece, none of the responsible agencies, the Federal Ministry of Health (FMoH), or the Yobe State Ministry of Health has made any public announcement, and there is nothing on any of their websites. What YOU need to do: If you are a clinician reading this, especially if you are practicing in northern Nigeria where the few cases have been reported, look out for the bottles in the image above and retrieve these. Immediately contact relevant authorities with the information. Please inform any clinician that you know who deals with children on the situation and ask them to look out for these bottles. Distribution chains are generally weak and porous in Nigeria, but we know how well we can work together when there are concerted efforts. Details of the product being withdrawn is as below:
Composition: Chlorhexidine Digluconate Solution BP 7.1%
Manufacturing Licence No.: KD-214
Batch No: Variable
Manufacturing Date: Variable
Expiring Date: Variable
NAFDAC No: None
Manufactured By: Galentic Pharma(India) Pvt Ltd, R- 673 MIDC, T.T.C., Rabale, Thane Belapur Road, Navi, Mumbai, India.
What you do need to know about Chlorhexidine Gel: Used appropriately, the evidence is that this gel, leads to a reduction in the risk of death by 23% in children when applied on the umbilical stump. We wrote this piece on Nigeria Health Watch highlighting the impact of its introduction in Bauchi and Sokoto States. Read here an excellent recently published article on the impact of the gel in Sokoto and lessons learnt from its introduction. The only licensed formulation of chlorhexidine in Nigeria is as a GEL in a TUBE; which is what is recommended and manufactured in Nigeria. Chlorhexidine is life saving when applied appropriately on the cord, but should not be applied on any other part of the body. Its appropriate use is described in the image below.
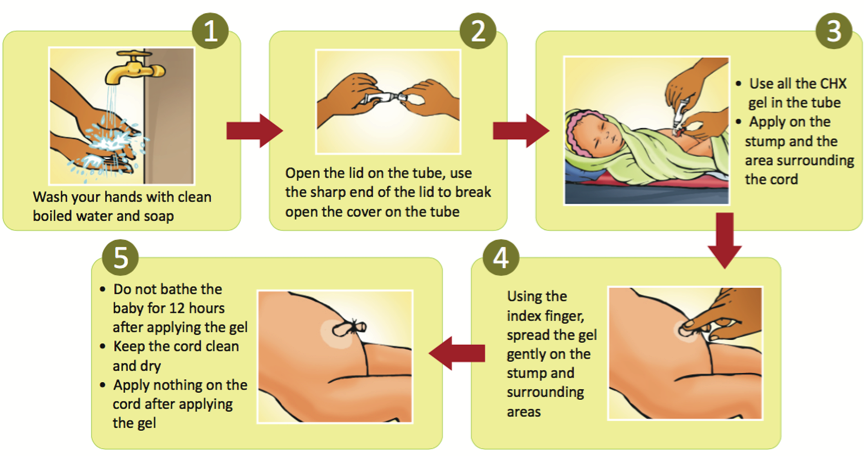
What are the unanswered questions?
- How can a drug, not registered by NAFDAC, not appropriately packaged, end up being distributed to mothers in government health care facilities? Who procured, imported, authorised, distributed this product? Where did the product governance processes fail?
- What assurance can be given to the Nigerian public that ALL of the bottles that were procured and distributed have been retrieved? The information on numbers procured, distributed and retrieved must be shared publicly to restore and assure public confidence.
- In 2014, the Nigerian Ministry of Health granted regulatory approval for the production Chlorhexidine Digluconate 7.1%, by a local company, Drugfield Pharmaceuticals Ltd. Why should this commodity be imported at all?
- What are the medico legal consequences for healthcare providers through the entire chain from clinicians to government to the regulators through to the manufacturers, considering the implications of a life without vision for the babies involved?
- How do we safeguard the legitimate use of lifesaving chlorhexidine gel in Nigeria.
Source:Nigerian Healthwatch