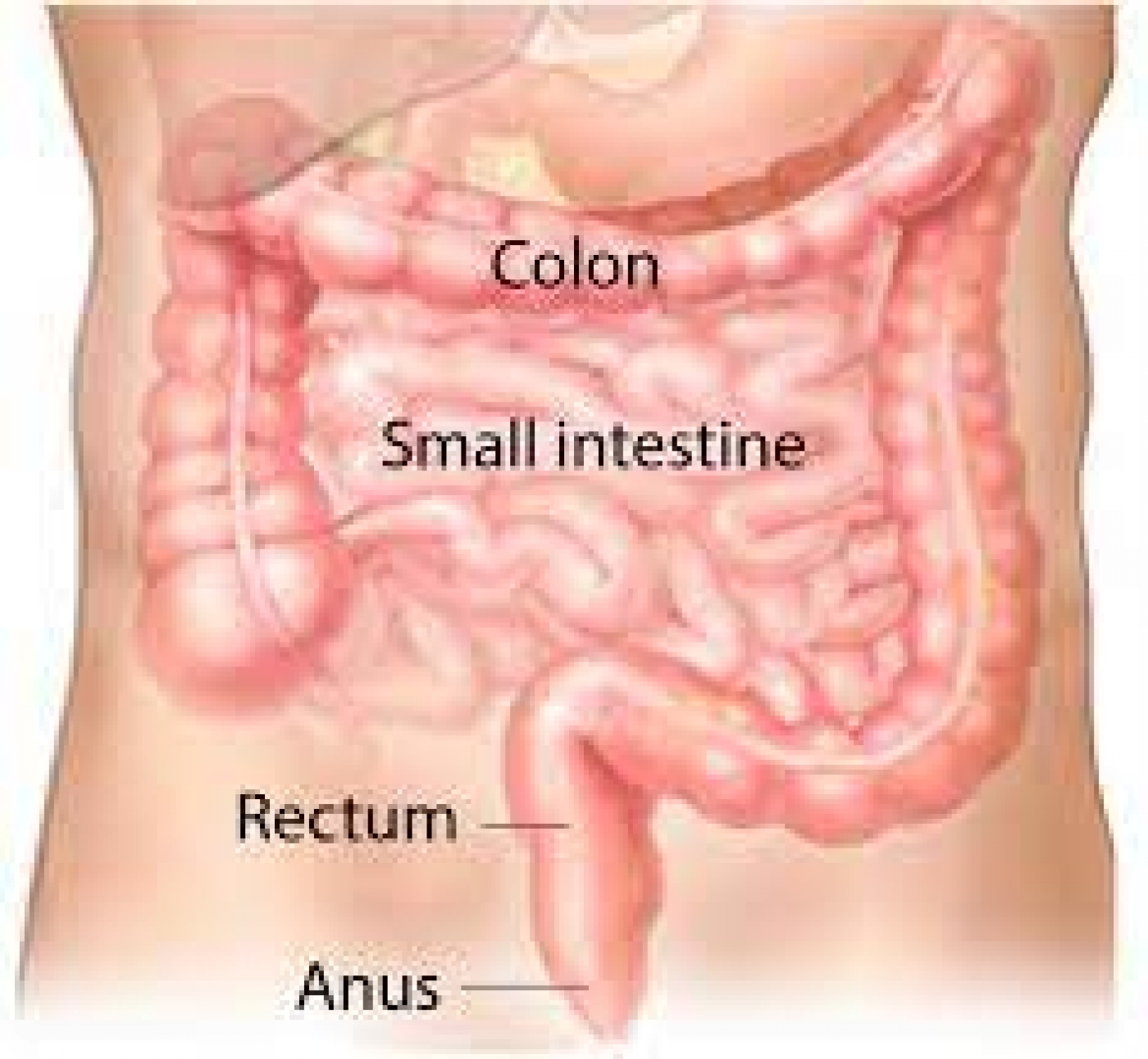
They reported that they were unable to detect any trace of cancer in the bodies of the study participants, even after carrying out a series of physical exams, endoscopies, PET scans, and MRI scans.
The study comprised 16 individuals who were administered Dostarlimab intravenously, at a dose of 500 mg every three weeks for half a year.
Dostarlimab is a monoclonal antibody used as a medication for the treatment of endometrial cancer. It is an experimental drug in the class of drugs called immune checkpoint inhibitors. It works by attaching to a protein called PD-1 on the surface of cancer cells, this aids the immune system to identify and attack cancer.
Of the 16 patients in the study, 12 completed treatment with Dostarlimab and have undergone at least six months of follow-up while the remaining four patients have received at least one dose of the drug and continue to receive treatment.
Patients were considered for the study only if they were 18 years and above. Other eligibility conditions included no previous receipt of immunotherapy, chemotherapy, or radiation for the rectal tumour and no active autoimmune disease and active infectious disease.
The doctors while noting that the study is investigator-initiated research, however, noted that a longer follow-up is required to gauge the duration of response.
According to the New York Times report, one of the authors of the study, Dr. Luis A. Diaz Jr of Memorial Sloan Kettering Cancer Center, said he was not aware of any other study in which a treatment totally removed cancer in all patients.
He said, “I believe this is the first time this has happened in the history of cancer.”
Source: healthwise